how many electrons is one coulomb|Electrons in a Coulomb : Manila How many electrons are there in 1 coulomb? Solution. Verified by Toppr. ∵ 1 electron contains 1.6 × 10 − 19 C of charge. ∴ 1 coulomb of charge is carried by 1 1.6 × 10 − 19 = . If you have won a prize of $50,000 (Match 4 + Powerball) or less, the amount will be multiplied by the value of the Power Play number if you have added this option to your ticket. Anyone who matches five numbers without the Powerball will see their award doubled from $1 million to $2 million, regardless of which Power Play number is .
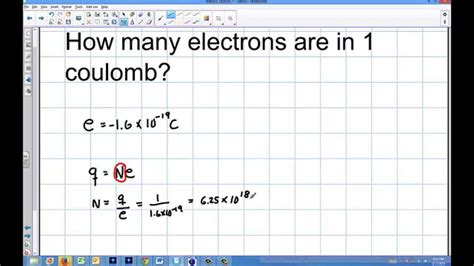
how many electrons is one coulomb,Solution. Step 1: Given data. To figure out how many electrons make up one coulomb of charge, e = 1 .6 × 10 − 19 C. ∴ q = 1 C. Step 2: Concept used. An electron's charge is negative and is 1.6 × 10 - 19 coulomb. Electrons constituting one coulomb of charge, .
How many electrons are there in 1 coulomb? Solution. Verified by Toppr. ∵ 1 electron contains 1.6 × 10 − 19 C of charge. ∴ 1 coulomb of charge is carried by 1 1.6 × 10 − 19 = .
The coulomb (symbol: C) is the unit of electric charge in the International System of Units (SI). It is equal to the electric charge delivered by a 1 ampere current in 1 second and is defined in terms of the elementary charge e, at about 6.241509×10 e.

Physics. Question. Calculate the number of electrons constituting one coulomb of charge. Solution. Verified by Toppr. we know that the magnitude of charge on one electron .how many electrons is one coulomb Electrons in a Coulomb Named for the 18th–19th-century French physicist Charles-Augustin de Coulomb, it is approximately equivalent to 6.24 × 10 18 electrons, with the charge of one electron, the .
I've just read that the unit of charge is the coulomb, and that 1C = 6.25 ×1018 1 C = 6.25 × 10 18 electrons, because charge on one electron is e = 1.602 .
One coulomb is equal to the amount of charge from a current of one ampere flowing for one second. One coulomb is equal to the charge on 6.241 x 10 18 protons. The charge on 1 .
Electrons in a Coulomb How do you calculate that one coulomb is equal to 6.24x10^18 electrons. The Coulomb is a ’derived’ unit. That is, its value depends on the basic units of the metric system, the .
The unit of electric charge in the metre–kilogram–second and SI systems is the coulomb and is defined as the amount of electric charge that flows through a cross section of a conductor in an electric circuit during each .The SI unit for electric charge is the coulomb (abbreviated as “C”), which is named after the French physicist Charles Augustin de Coulomb, who studied the force between charged .How many electrons are there in one coulomb of electricity? 6.023 .

For example, in the reaction Ag + (aq) + e − → Ag(s), 1 mol of electrons reduces 1 mol of Ag + to Ag metal. In contrast, in the reaction Cu 2 + (aq) + 2e − → Cu(s), 1 mol of electrons reduces only 0.5 mol of Cu 2 + to Cu metal. Recall that the charge on 1 mol of electrons is 1 faraday (1 F), which is equal to 96,486 C.
how many electrons is one coulombFor example, in the reaction Ag + (aq) + e − → Ag(s), 1 mol of electrons reduces 1 mol of Ag + to Ag metal. In contrast, in the reaction Cu 2 + (aq) + 2e − → Cu(s), 1 mol of electrons reduces only 0.5 mol of Cu 2 + to Cu metal. Recall that the charge on 1 mol of electrons is 1 faraday (1 F), which is equal to 96,486 C.A single electron carries a charge of q e − = −1.602 × 10 −19 C q e − = −1.602 × 10 −19 C. Dividing the net charge of the ink droplet by the charge q e − q e − of a single electron will give the number of electrons captured by the ink droplet. Transcript. Question 3 Page 200 Calculate the number of electrons constituting one coulomb of charge. We know that Charge on 1 Electron = 1.6 × 10−19 C 1 Electron = 1.6 × 10−19 C 1.6 × 10−19 C = 1 Electron 1 C = 1/1.6 × 10−19 electrons 1 C = 10/16 × 1019electrons 1 C = 5/8 × 1019 electrons 1 C = 0.625 × 1019 electrons 1 C = .Electron charge is equal to the charge of an electron, and is the inverse of elementary charge, which is the magnitude of the charge of a proton. It is equal to 1.602176634×10−19 coulombs, per the 2019 SI redefinition of the coulomb. Electron charge can be abbreviated as e; for example, 1 electron charge can be written as 1 e.What is a coulomb (C)? A coulomb (C) is the standard unit of electric charge in the International System of Units ().It is the amount of electricity that a 1-ampere current carries in one second (s).A quantity of 1 C is equal to the electrical charge of approximately 6.24 x 10 18 electrons or protons. This comes to about 6.24 quintillion particles.. In the SI .Compare the electrostatic force between an electron and proton separated by 0. 530 × 10 − 10 m 0. 530 × 10 − 10 m with the gravitational force between them. This distance is their average separation in a hydrogen atom. Strategy. To compare the two forces, we first compute the electrostatic force using Coulomb’s law, F = k | q 1 q 2 | r . e denotes the elementary charge, approximately 1.602176634 × 10^-19 C. This formula allows users to effortlessly calculate the electric charge in Coulombs by multiplying the number of electrons by the elementary charge constant.If 1 electron carries a charge of 1. 602 × 10-19 coulombs, what will be the charge of 1 mole of electrons? Q. which is bigger a coulomb or charge on an electron how many electronic charges form one coulomb of chargeCoulomb: Coulomb is the unit used to quantify charge. 1 Coulomb is equivalent to the charge carried by {eq}1.6\times 10^{-19} {/eq} protons. Let us test our understanding by going over two .The charges in Coulomb’s law are q 1 = q 2 = q ink drop, q 1 = q 2 = q ink drop, so the numerator in Coulomb’s law takes the form q 1 q 2 = q ink drop 2 q 1 q 2 = q ink drop 2. Inserting this into Coulomb’s law and solving for the distance r givesOne coulomb consists of 6.24 × 10 18 natural units of electric charge, such as individual electrons or protons. From the definition of the ampere, the electron itself has a negative charge of 1.602176634 × 10 −19 coulomb. An electrochemical unit of charge, the faraday, is useful in describing electrolysis reactions, such as in metallic .To convert a measurement in coulombs to a measurement in electron charge, multiply the electric charge by the following conversion ratio: 6.2415E+18 electron charge/coulomb. Since one coulomb is equal to 6.2415E+18 electron charge, you can use this simple formula to convert: electron charge = coulombs × 6.2415E+18
One coulomb of charge is a lot of charge, so much that, two particles, each having a charge of +1 C and separated by a distance of 1 meter exert a force of \(9\times 10^9 N\), that is 9 billion newtons on each other. . typically electrons, from one object to the other. One point that we did not make in the discussion above is that charge is .When 1 Coulomb charge flow through a wire in 1 second then the current through the wire is 1 AMPERE. I=Q/t. 1 Ampere = 1 Coulomb /1 Second Charge on 1 electron = 1.6 x 10^-19 Coulomb By unitary method, If 1.6 x 10^-19 Coulomb / Second (Ampere) = Current by 1 electron then, 1( Coulomb / Second) or (Ampere) = 1 / (1.6 x 10^-19) electrons i.e, 6. .
Law). Charge is a scalar and is measured in coulombs 1. The coulomb is actually defined in terms of electric current (the flow of electrons), which is measured in amperes2; when the current in a wire is 1ampere, the amount of charge that flows past a given point in the wire in 1 second is 1 coulomb. Thus, 1ampere = 1A = 1 C s.
how many electrons is one coulomb|Electrons in a Coulomb
PH0 · electrostatics
PH1 · How many electrons are there in 1 coulomb?
PH2 · Electrons in a Coulomb
PH3 · Electric charge
PH4 · Coulomb
PH5 · Calculate the number of electrons constituting one coulomb of charge
PH6 · Calculate the number of electrons constituting one coulomb of cha
PH7 · Calculate the number of electrons constituting one coulomb of
PH8 · Calculate the number of electrons constituting one coulomb of
PH9 · 18.1 Electrical Charges, Conservation of Charge, and